Unstable atoms tend to become stable by changing the structure of their nucleus and emitting excess energy as radiation.
The ability of an atomic nucleus to spontaneously decay with the emission of elementary particles, gamma rays, and/or nuclear fragments is called radioactivity, and the atom is considered to be radioactive.
The radioactive isotope is called a radionuclide.
Radioactive decay (from lat. radius "ray" and aktīvus "effective") is a spontaneous changing of the composition of unstable atomic nuclei (charge Z and mass number A) with the emission of elementary particles, gamma rays and/or nuclear fragments.
The initial nucleus is called the mother, or parent nucleus. The correspondent radionuclide is also called the mother (parent) one. The nucleus that appears because of radioactive transformation is called the daughter nucleus, or progeny nucleus. The same name the resulting radionuclide has. Mother and daughter nuclei and the radionuclides composed from them are called being genetically connected. If the daughter nucleus is radioactive, they talk about radioactive transformation chains, or radioactive series (families). All elements within radioactive series are also called genetically connected ones.
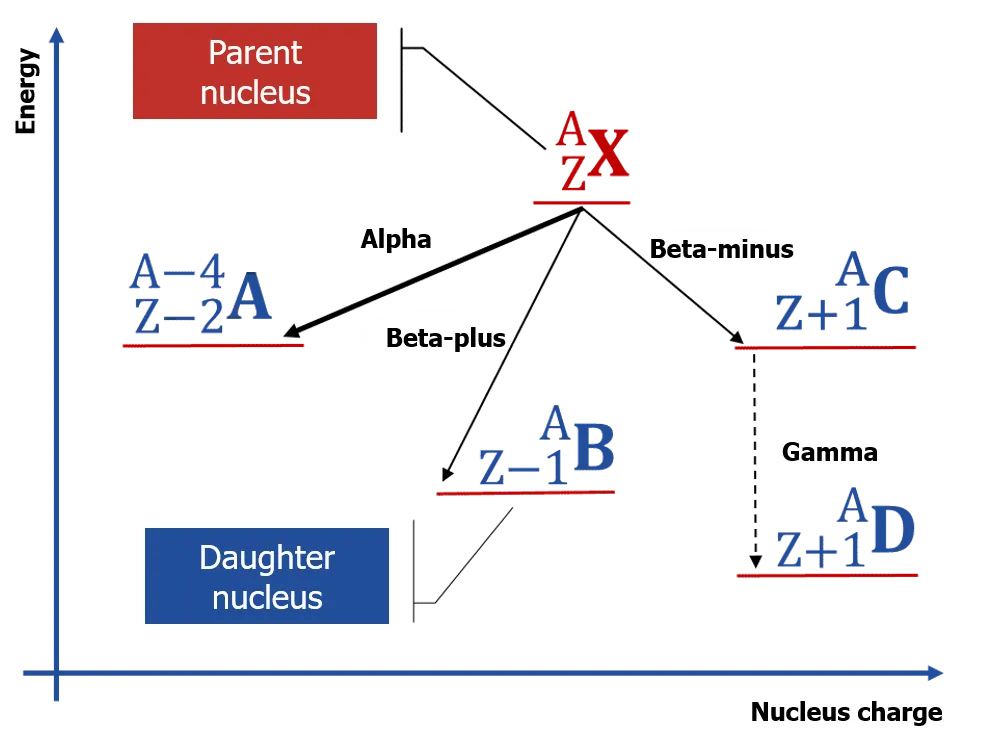
The basic radioactive phenomena are a-decay, ß± -transformations (decays), and γ-radiation.
The following spontaneous processes are related to beta-transformations:
- ß--transformation (ß--decay) is the spontaneous emission of the pair "electron and electronic antineutrino" by a nucleus,
- ß+- transformation (ß+-decay) is the spontaneous emission of the pair "positron and electronic neutrino" by a nucleus,
- е-capture, electronic capture (e) is the phenomenon of spontaneous capture of an electron from an atomic electron shell (more often from К-shell; that is why the term К-capture is frequently used) accompanying by emission of the electronic neutrino,
- g-radiation (g) is the process of spontaneous emission by a nucleus of a photon (several photons) having energies referred to the ionizing radiation.
Transitions of nuclei from long-living states (with an average lifetime of more than 100 ns) to lower energy levels are called isomeric transitions (IT), and nuclei having long-living states are called isomers. The isomers’ excitation can be released not only due to electromagnetic interactions but also by emission of alpha-particles, beta-particles, or other ones at some conditions likely taken place.
Except for these processes, the following phenomena are referred to the radioactivity phenomena:
- spontaneous fission (denoted by f from the word fission) is the spontaneous fission of heavy nuclei onto two fragments having comparable masses (seldom there are three or even more of them),
- cluster activity is the spontaneous emission of nuclei heavier than 4He named nuclei-clusters (the clusters up to 32S are discovered for today),
- neutron activity (n) is the spontaneous emission of neutrons by nuclei (it takes place among light nuclei overloaded by neutrons, e.g. 5He, or 10Li),
- proton activity (p) is the spontaneous emission of protons by nuclei (e.g., 112Cs, 135Tb; the most heavy is 185Bi),
- beta-delayed transformations are the spontaneous emission of neutrons (ßn,ß2n), protons (ßp, ep, e2p), alpha-particles (ßa, ea) by nuclei, or beta-delayed spontaneous fission of super heavy nuclei (ßbf, ef). They take place at highly excited states of daughter nuclei, occurring in beta-transformations. A typical example is the emission of delayed neutrons by heavy nuclei fission fragments.

Sometimes, the following additional beta-transformation processes are indicated.
- Beta-transformation of "bare" nuclei is the spontaneous ß--transformation in a highly ionized atom having a half-life different (sometimes, quite different) from the half-life of the corresponding neutral atom. For example, the half-life of 187Re in a neutral atom is 5×1010 years, but for the ionized 187Re, it is less by 9 degrees. In multi-charged radioactive ions, there is bound beta-transformation, that is spontaneous emission by nuclei of the pair "electron – antineutrino" with the consequent capture of the emitted electron by an electron shell of the atom (denoted bb).
- Doubled beta-transformations are the emission of two pairs of "electron –antineutrino" (2ß-), two pairs of "positron – neutrino" (2ß+), or doubled electronic capture (2e), or electron capture with positron emission (eß+). These processes are very rare and have the most long half-lives that are known today (from 7×1018 years for 100Ru to (3,5±2,0)×1024 years for 128Te).
Rigorously saying, the fission fragments, alpha-particles, clusters, protons, and neutrons that appeared in different kinds of radioactivity phenomena can be considered as compartments of atomic nuclei but with some clauses (one should consider them as virtual particles). Electrons and antineutrinos as well as positrons and neutrinos are not the nucleus compartments and also are not the nucleon compartments. Gamma-radiation of nuclei happens as a result of electromagnetic transitions in nuclei, and, therefore, they are not to be referred to the nuclear decays. Thus, the term “decay” habitual in scientific and public use is not worth treating literary to the radioactivity phenomenon, but it should be considered as a synonym of the term “transformation”. In such a sense, the terms “beta-decay”, and “gamma-decay” should be treated.
Electromagnetic quantum transitions in nuclei may be accompanied by internal conversion (an atom emits an electron from its electron shell except for emitting a photon, Fig. 6), or pair conversion (the forming of electron-positron pair except gamma-quantum). However, these phenomena are not considered separate kinds of radioactivity.


Thus, radioactivity should be treated as an atomic phenomenon linked with atomic nuclear transformations.
Some radionuclides demonstrate two (e.g., a- and ß- of 212Bi, ß- and e of 40K and 64Cu, p and a of 185Bi) and even three kinds of radioactivity (e.g., a, e and ep of 110Xe, ß-, ß-n and ß-2n of 98Rb, e, ep and e2p of 35Ca), happened with different likelihood. In these cases, they say that such radionuclides can undergo radioactive transformations along different modes.
| Series | Most long living radionuclide | Half-Life |
|---|---|---|
| A = 4n | 23290Th | 1.41*1010y |
| A = 4n+1 | 23793Np | 2.14*106y |
| A = 4n+2 | 23892U | 4.5*109y |
| A = 4n+2 | 23592U | 7*108y |
